American Amnion
A Natural Advantage
Our BioRetain process is designed to preserve more growth factors and cytokines with each American Amnion allograft—delivering a minimally-manipulated covering.

American Amnion AC is a human connective tissue matrix comprised of amnion/chorion tissue. It is intended as a covering for homologous use.
American Amnion AC is manufactured using our proprietary BioRetain process. This creates a dehydrated human amniotic membrane (DHACM) allograft.

American Amnion is a human connective tissue matrix comprised of amnion tissue. It is intended as a covering for homologous use.
American Amnion is manufactured using our proprietary BioRetain process. This creates a dehydrated human amniotic membrane (DHAM) allograft.

Easy to Use—Easy to Store
Allografts
- are held in place via hydrostatic tension and may be used in an open incision or placed as a dermal substitute*.
- are aseptically processed and terminally sterilized via e-beam irradiation.
- have a 4-year shelf life and can be stored at ambient temperatures.
*Standard fixation can be used. Reference American Amnion instructions for use.
The Intrinsic Properties of Dehydrated Human Amnion/Chorion Membrane
make American Amnion a versatile option for a wide variety of topical covering applications.
Properties of Dehydrated Human Amnion/Chorion Membrane

Provides barrier for protection
Terminally sterilized

Helps
prevent
moisture loss
Provides a
structure for
tissue integration

Contains a
range of
growth factors

Naturally contains cytokines
Amniotic tissue is a natural reservoir of growth factors, extracellular matrix (ECM) components, and cytokines known to support the body’s natural healing processes.
Multi-layered Support
Placentally-derived human amniotic membrane (AM) is a potent source of growth factors and anti-inflammatory cytokines and has successfully been used in regenerative medicine for over a century. Early users of AM for wounds and post-surgical applications noted how the membrane seemed to disappear and integrate with the patient’s own tissue without a host reaction. This apparent immune neutrality is a result of mechanisms that suppress and modulate the immune system.
The use of AM was initially limited due to storage challenges associated with use of the fresh tissue. Modern processing methods – including dehydration – have delivered options that have longer shelf lives, can be stored at ambient temperatures, and can be terminally sterilized. In recent decades, advances in our understanding of placental tissues and improved processing abilities have resulted in the creation of dehydrated combined amniotic/chorionic membrane (DHACM) products.
DHACM is now widely used where clinicians believe a thicker covering, richer in pro-healing factors, is needed. Post-surgical, trauma, burn, and complex wound applications for DHACM have been well-documented in the literature, and new applications continue to be explored.
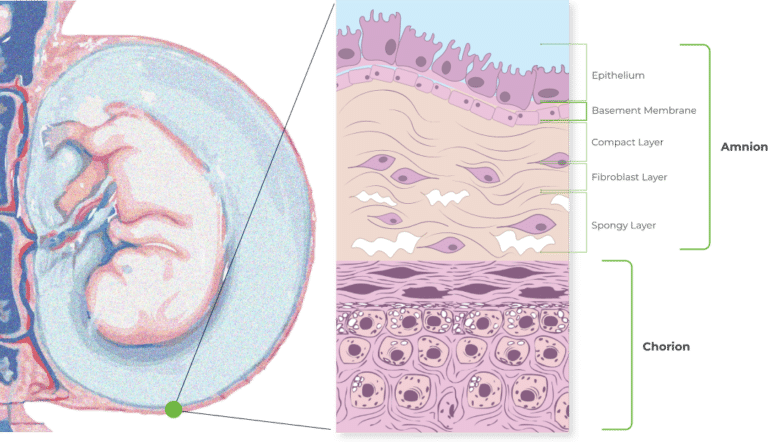
The Combined Amniotic and Chorionic Membranes
The combination of amnion and chorion is thicker and higher in tensile strength than allografts containing amnion alone. It is also richer and more varied in growth factors as well thanks to the higher quantity of growth factors present in the chorion layer.
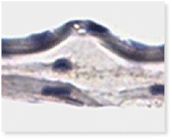
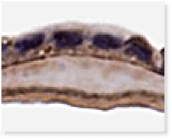
A Look Inside American Amnion AC
Why Retain the Intermediate Layer?
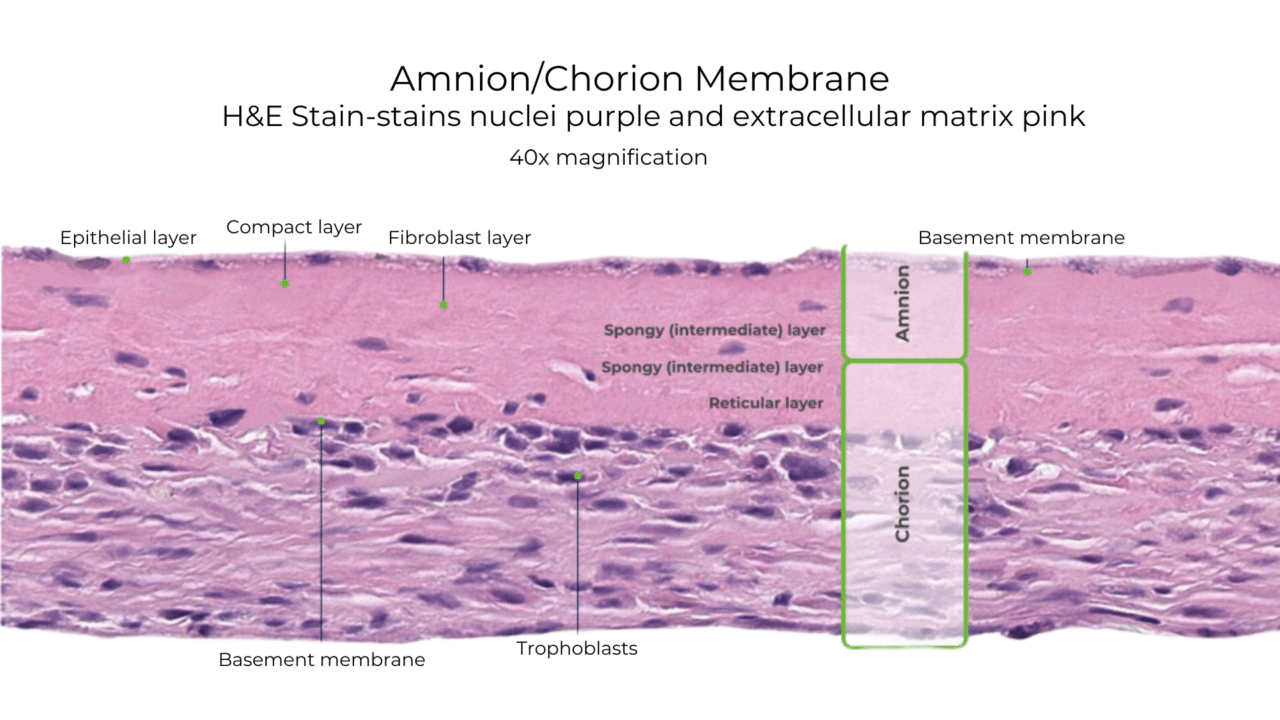
Unlike some other DHACM products, American Amnion AC also preserves the intermediate layer between the amnion and chorion. The intermediate layer has its own components, including chemokines, growth factors, interleukins, and protease inhibitors.
Natural Amniotic and Chorionic Components Retained in American Amnion
American Amnion AC allografts are prepared using our proprietary BioRetain process, which was designed to help preserve the natural ECM components, cytokines, and endogenous growth factors within fresh tissue.
The following substances were isolated within American Amnion AC allografts (n=6)*:
Cytokines:
- IL-1 receptor antagonist
This substance naturally inhibits pro-inflammatory effects of IL-1.
Collagens I, III, IV, V, and VI
- Elastin
- Laminin
- Fibronectin
- Proteoglycans
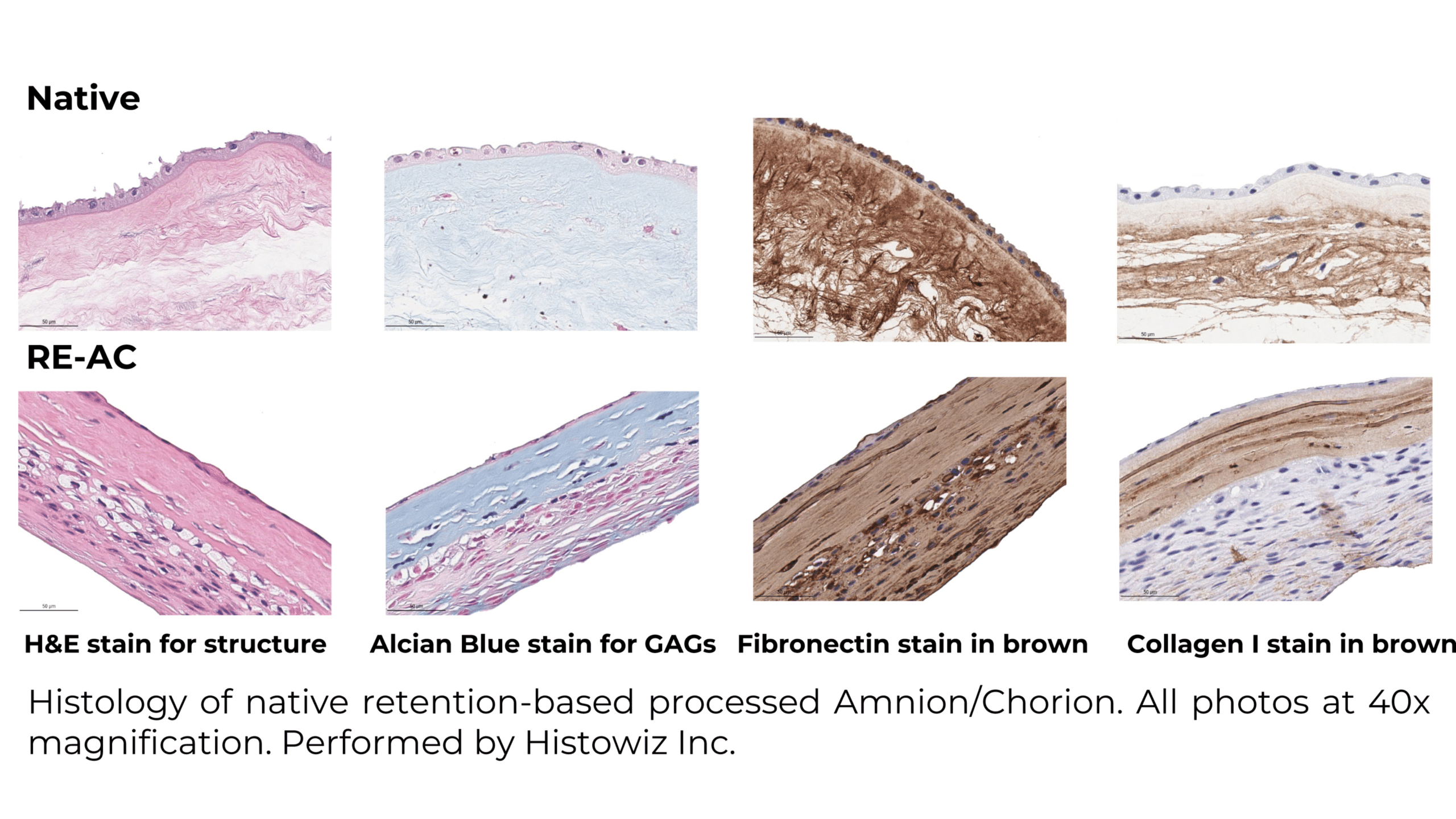
Assessing Treatment Efficiency in Diabetic Foot Ulcers: Retention Processed* Amnion/Chorion Membranes vs. SOC: A Retrospective Analysis
Abstract
Diabetic foot ulcers are a severe complication for diabetic patents, significantly impacting patient quality of life and healthcare systems efficiency. These ulcers often lead to hospitalization and amputation. Traditional Standard of Care (SOC), are inadequate for many patients, necessitating the use of advanced wound care products, such as human placental membranes. This study conducts a retrospective analysis to compare the effectiveness of a human placental amnion/chorion membrane product using retention-based processing (RE-AC) and Standard of Care (SOC) in the treatment modality for chronic diabetic foot ulcers (DFUs).
Methods/Statistical Analysis
The study collected retrospective observational data from electronic health records (EHRS) of patients treated with retention based processed Amnion/Chorion* (RE-AC) as a wound covering at three outpatient wound care centers. Additionally, synthetic control SOC patients were matched from a wound registry using Coarsened Exact Matching (CEM). Patients were categorized into two cohorts based on whether they received RE-AC or SOC.
Key metrics included wound size progression and wound closure. The analysis employed Bayesian Regression and Hurdle Gamma Analysis of Variance (ANCOA) models. These methods allow for the highest statistical power of analyses that are focused on change form a baseline and increase statistical power and precision. They are a more robust approach and offer the opportunity to control potential confounding variables, thereby enhancing the precision of our findings.
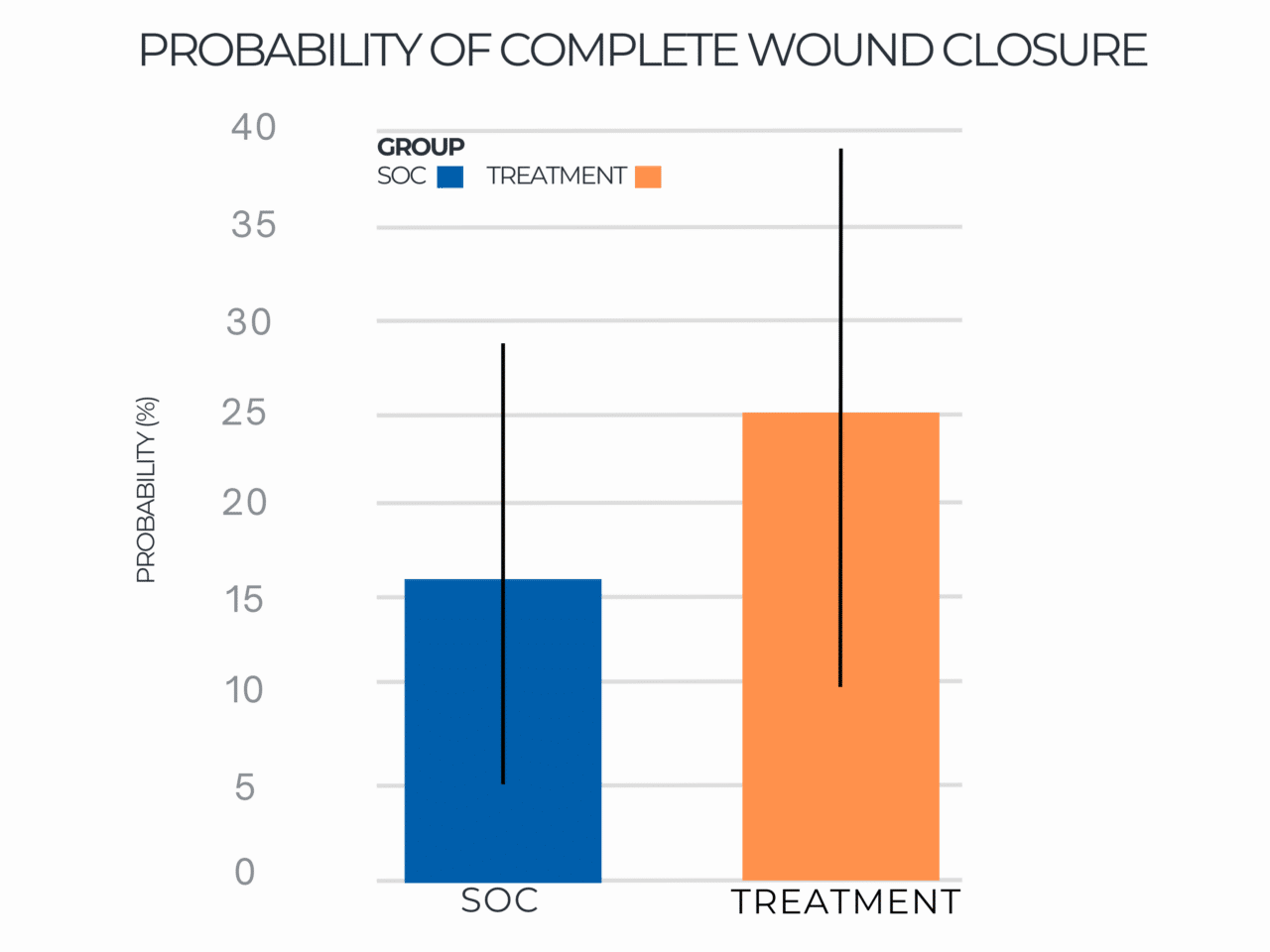
Probability of Complete Wound Closure
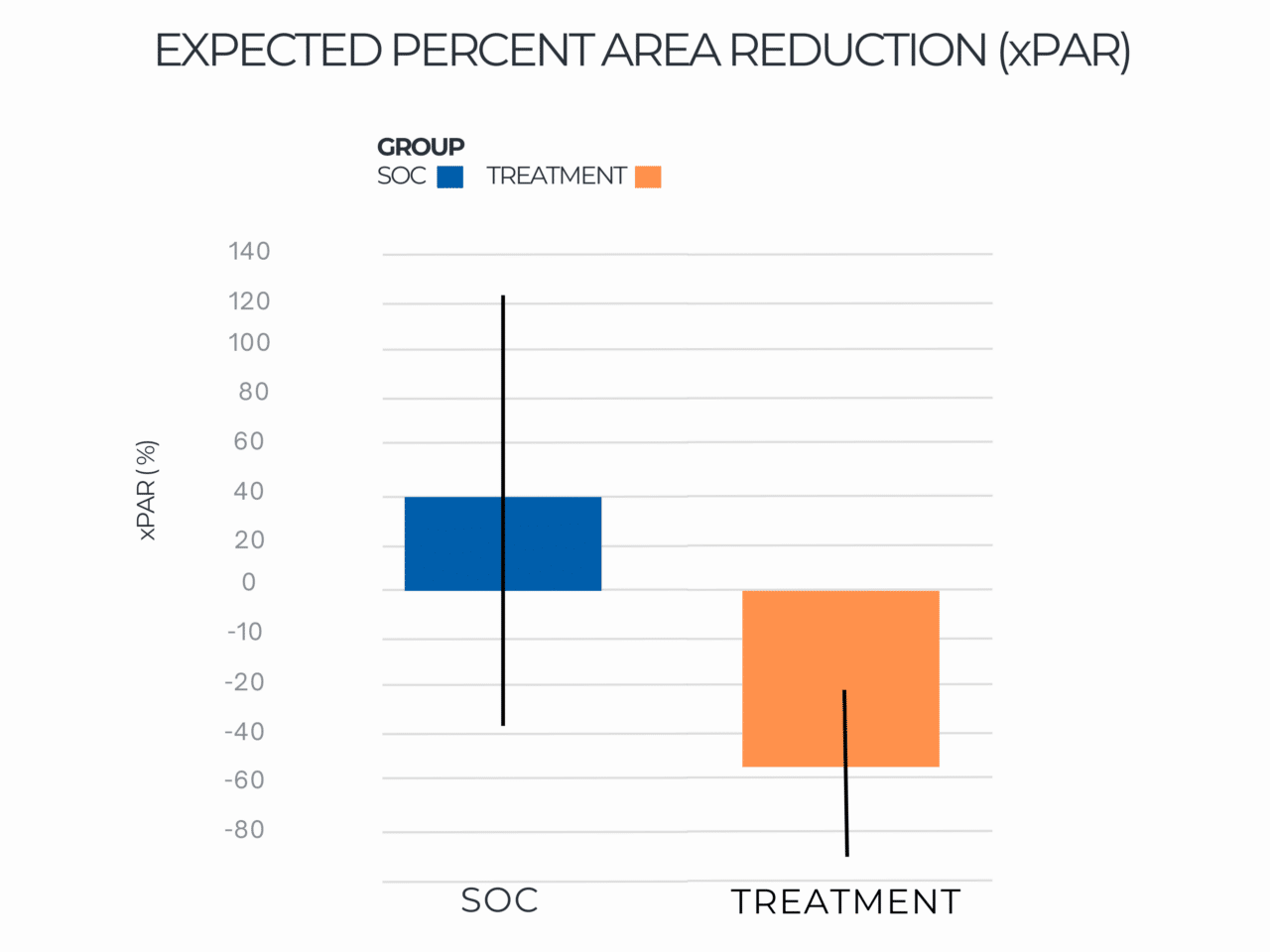
Expected Percent Area Reduction (xPAR)
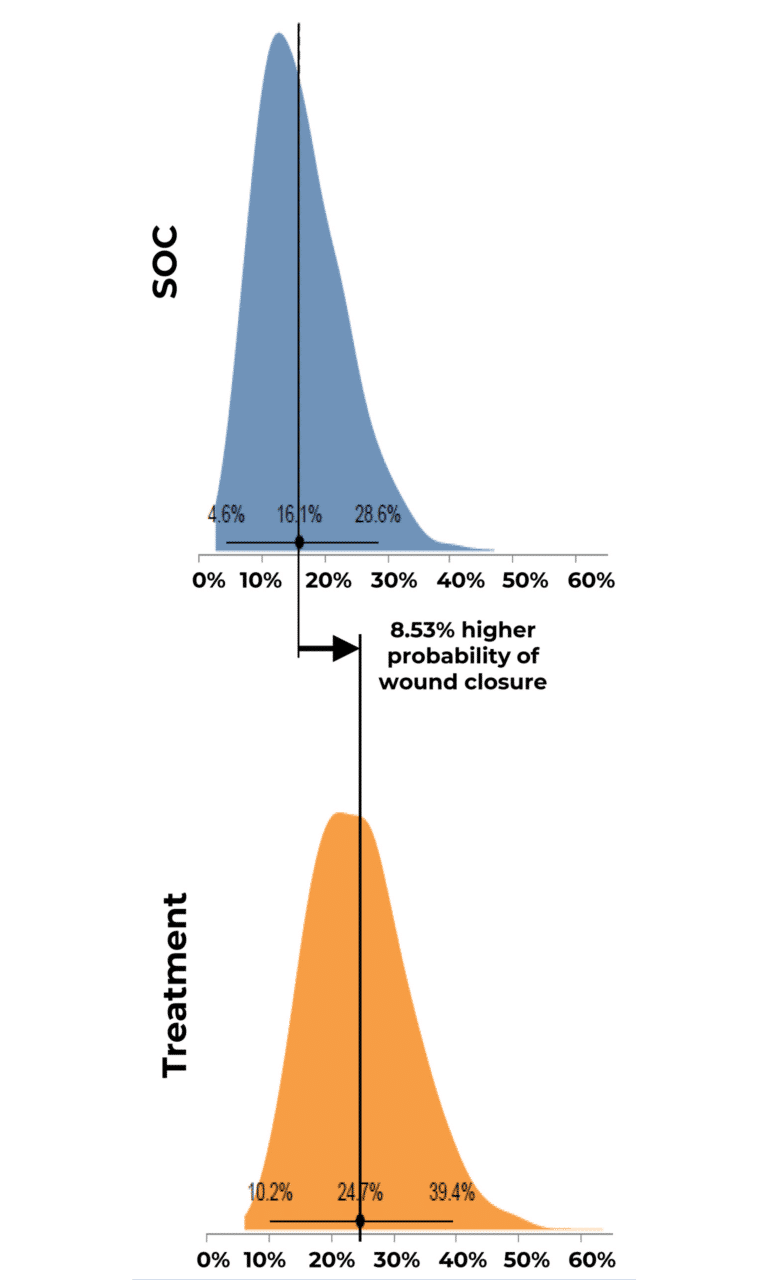
Probability of Complete Wound Closure by Group Distribution
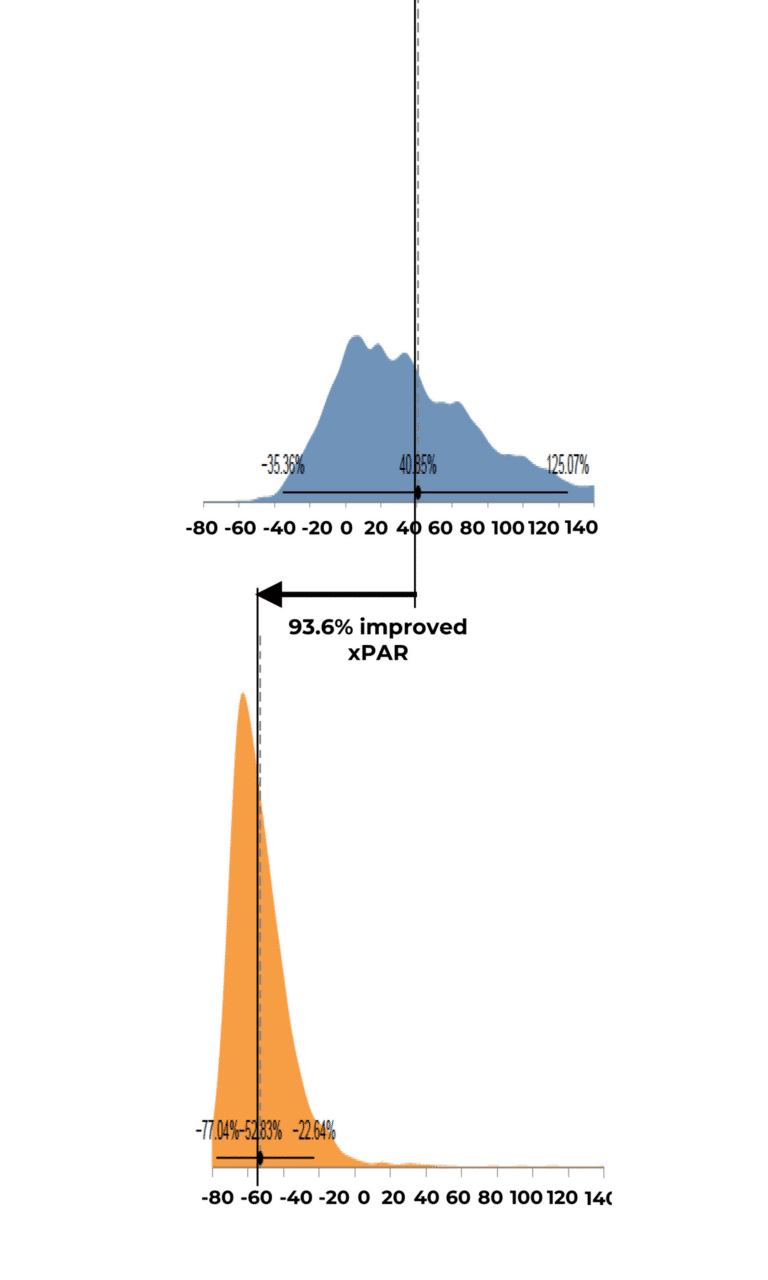
Expected Percent Area Reduction by Group Distribution
Histology of American Amnion AC
Hyaluronic acid (HA):
Average HA concentration in a BioRetain allograft: 48,355,062 picograms/cm2
—nearly 8 times higher than our single-layer allograft
Growth factors:
- Epidermal growth factor (EGF)
- Vascular endothelial growth factor (VEGF)
- Basic fibroblast growth factor (bFGF)
- Keratinocyte growth factor (KGF)
The chorionic layer provides a significantly higher amount of growth factors than the amniotic layer, with the exception of EGF.
*This is not a comprehensive list of the substances found within an American Amnion AC allograft.
Ordering Information – American Amnion AC
American Amnion AC allografts are available in a wide range of sizes and shapes for maximum versatility.

| Product SKU | Size |
| 300-004-0202-010 | 2 x 2 cm |
| 300-006-0203-010 | 2 x 3 cm |
| 300-018-0303-010 | 3 x 3 cm |
| 300-016-0404-010 | 4 x 4 cm |
| 300-025-0406-010 | 4 x 6 cm |
| 300-032-0408-010 | 4 x 8 cm |
| 300-049-0707-010 | 7 x 7 cm |
Ordering Information – American Amnion
American Amnion allografts are available in a wide range of sizes and shapes for maximum versatility.

| Product SKU | Size |
| 200-004-0202-010 | 2 x 2 cm |
| 200-008-0204-010 | 2 x 4 cm |
| 200-016-0404-010 | 4 x 4 cm |
| 200-025-0406-010 | 4 x 6 cm |
| 200-049-0707-010 | 7 x 7 cm |
| 200-254-0015-010 | 15mm |
Contact our product experts for more information or to place an order:
Product Hotline:
Local: 954-380-8342
Toll-Free: 1-888-948-BSEM (2736)
Email Inquiries:
orders@biostemtech.com
References
American Amnion is intended for homologous use as a covering and barrier for human tissue. Refer to American Amnion instructions for Use for information on safety, usage, and storage.
- American Amnion Instructions for Use
- Data on File
- Tenehaus M. The Use of Dehydrated Human Amnion/Chorion Membranes in the Treatment of Burns and Complex Wounds. Ann Plast Surg. 2017;78: S11–S13.
- Silini AR, Cargnoni A, Magatti M, Pianta S and Parolini O (2015) The long path of human placenta, and its derivatives, in regenerative medicine. Front. Bioeng Biotechnol.
2015;3:162. - Ramuta TZ, Šket T, Erjavec MS, Kreft ME. Antimicrobial Activity of Human Fetal Membranes: From Biological Function to Clinical Use. Front Bioeng Biotechnol. 2021;9:691522.
- Heckmann N, Auran R, Mirzayan R. Application of Amniotic Tissue in Orthopedic Surgery. Am J Orthoped. 2016;45(7):E421-E425.
- Niknejad H, Peirovi H, Jorjani M et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cells Mater. 2008;15(15):88-99.
- Moore MC, Bonvallet PP, Damaraju SM, et al. Biological characterization of dehydrated amniotic membrane allograft: Mechanisms of action and implications for wound care. J
Biomed Mater Res. 2020;1–8. - Wassmer C-H. Berishvili E. Immunomodulatory Properties of Amniotic Membrane Derivatives and Their Potential in Regenerative Medicine. Curr. Diab. Rep. 2020;20(31).
- Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol. 2014;5(23).
- Moreno SE, Massee M, Koob TJ. Dehydrated Human Amniotic Membrane Inhibits Myofibroblast Contraction through the Regulation of the TGFb‒SMAD Pathway In Vitro. JID
Innov. 2021;1:100020 - Zelen CM, Serena TE, Denoziere G, Fetterolf DE.A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic
foot ulcers. Int Wound J. 2013;10(5):502-507.
VENDAJE®, VENDAJE AC® and VENDAJE OPTIC® are perinatal tissue-derived allografts. Each product is designated as a Human Cell, Tissue, and Cellular and Tissue-Based Product (HCT/P) by the U.S. Food and Drug Administration (FDA), minimally manipulated, and produced in accordance with the FDA regulations for Good Tissue Practices (21 CFR 1270, 1271) in our AATB® accredited lab.