
AmnioWrap2® allografts are Intended for Homologous use as a Protective Covering
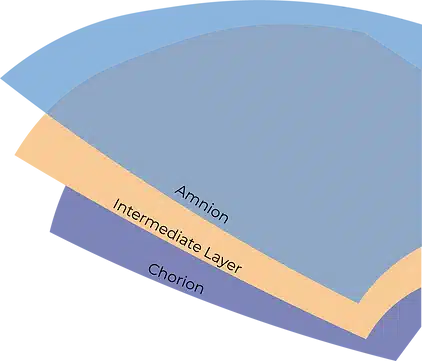
AmnioWrap2® is a placental-based allografts comprised of unseparated amnion and chorion membranes including the intact intermediate layer. The allografts are made from amniotic tissue that contain collagen extracellular matrix and a wide array of regulatory proteins including growth factors, cytokines, and chemokines.
AmnioWrap2 allografts act as a protective covering when placed over the wound bed or surgical site, providing the key components found in human placental tissues including an intact extracellular matrix (ECM), growth factors and cytokines.
Click here for more information and to place an order.
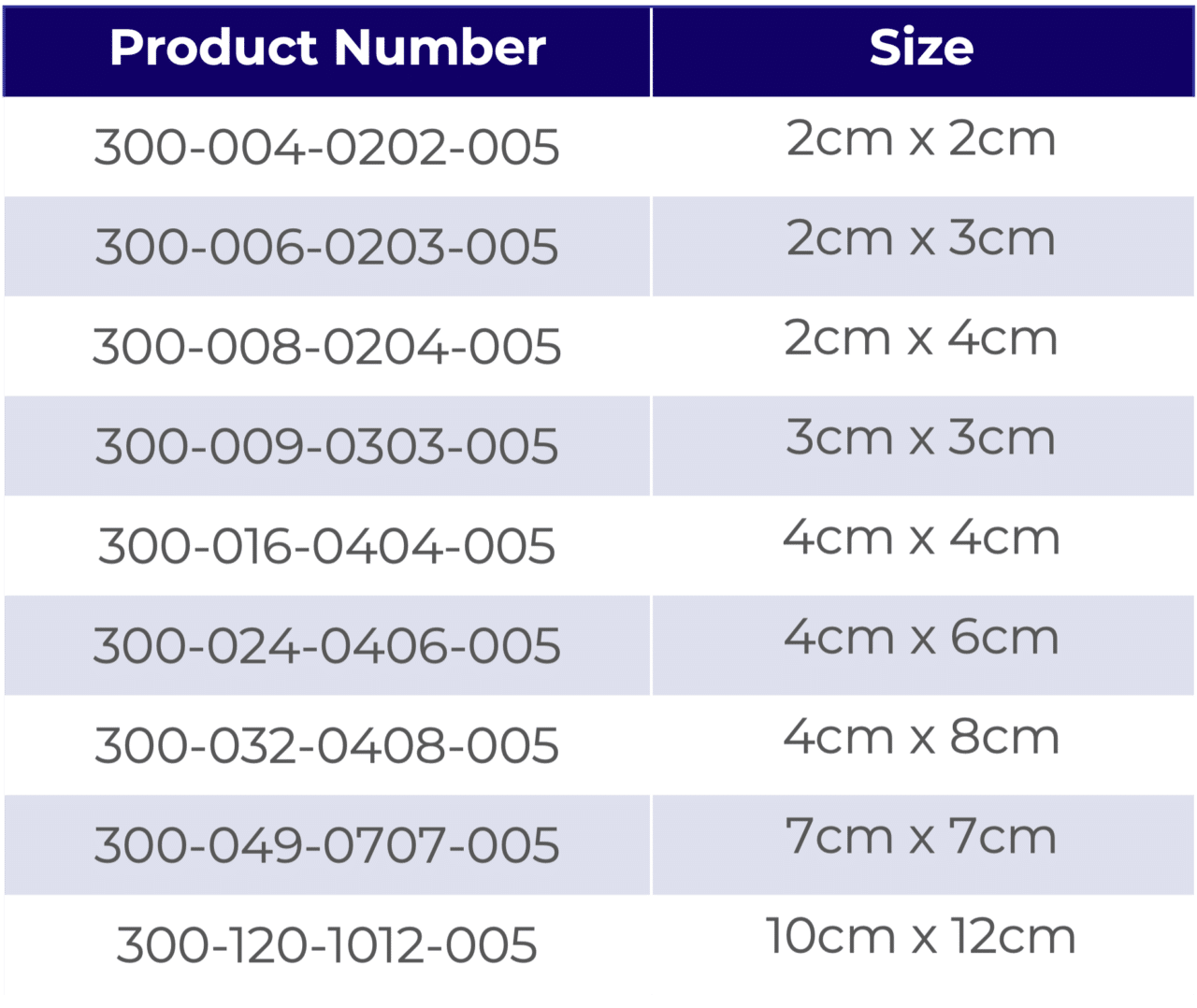
AmnioWrap2 Assortment:
Ideal for acute and chronic wound application.
(Tri-Layer — 250 microns thick)
HCPC code: Q4221
Click here for more information and to place an order.
AmnioWrap2® is intended for homologous use as a covering and barrier for human tissue. Refer to Instructions for Use for information on safety, usage, and storage.
References
- AmnioWrap2® Instructions for Use
- Data on File
- Tenehaus M. The Use of Dehydrated Human Amnion/Chorion Membranes in the Treatment of Burns and Complex Wounds. Ann Plast Surg. 2017;78: S11–S13.
- Silini AR, Cargnoni A, Magatti M, Pianta S and Parolini O (2015) The long path of human placenta, and its derivatives, in regenerative medicine. Front. Bioeng Biotechnol.
2015;3:162. - Ramuta TZ, Šket T, Erjavec MS, Kreft ME. Antimicrobial Activity of Human Fetal Membranes: From Biological Function to Clinical Use. Front Bioeng Biotechnol. 2021;9:691522.
- Heckmann N, Auran R, Mirzayan R. Application of Amniotic Tissue in Orthopedic Surgery. Am J Orthoped. 2016;45(7):E421-E425.
- Niknejad H, Peirovi H, Jorjani M et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cells Mater. 2008;15(15):88-99.
- Moore MC, Bonvallet PP, Damaraju SM, et al. Biological characterization of dehydrated amniotic membrane allograft: Mechanisms of action and implications for wound care. J
Biomed Mater Res. 2020;1–8. - Wassmer C-H. Berishvili E. Immunomodulatory Properties of Amniotic Membrane Derivatives and Their Potential in Regenerative Medicine. Curr. Diab. Rep. 2020;20(31).
- Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol. 2014;5(23).
- Moreno SE, Massee M, Koob TJ. Dehydrated Human Amniotic Membrane Inhibits Myofibroblast Contraction through the Regulation of the TGFb‒SMAD Pathway In Vitro. JID
Innov. 2021;1:100020
VENDAJE®, VENDAJE AC®, VENDAJE OPTIC® and AmnioWrap2® are perinatal tissue-derived allografts. Each product is designated as a Human Cell, Tissue, and Cellular and Tissue-Based Product (HCT/P) by the U.S. Food and Drug Administration (FDA), minimally manipulated, and produced in accordance with the FDA regulations for Good Tissue Practices (21 CFR 1270, 1271) in our AATB® accredited lab.
