VENDAJE®
A Natural Advantage
Our BioREtain process is designed to preserve more growth factors and cytokines with each VENDAJE allograft—delivering a minimally-manipulated covering.
VENDAJE® is Intended for Homologous use as a Protective Covering.
VENDAJE® is a human connective tissue matrix comprised of amniotic tissue
which is manufactured using our proprietary BioREtain process. This creates a dehydrated human amniotic membrane (DHAM) allograft.

Easy to Use—Easy to Store
Allografts
- are held in place via hydrostatic tension and may be used in an open incision or placed as a dermal substitute*.
- are aseptically processed and terminally sterilized via e-beam irradiation.
- have a 4-year shelf life and can be stored at ambient temperatures.
*Standard fixation can be used. Reference VENDAJE instructions for use.
The Intrinsic Properties of Dehydrated Human Amniotic Membrane (DHAM)
make VENDAJE® a versatile option for a wide variety of topical covering applications.
Properties of Dehydrated Human Amniotic Membrane

Provides barrier for protection
Terminally sterilized

Helps
prevent
moisture loss
Provides a
scaffolding for
tissue integration

Contains a
range of
growth factors

Naturally contains cytokines
Amniotic tissue is a natural reservoir of growth factors, extracellular matrix (ECM) components, and cytokines which are known to support the body’s natural healing processes.
A Natural Foundation for Wound Care
Placentally-derived human amniotic membrane (AM) is a potent source of growth factors and cytokines and has successfully been used in regenerative medicine for over a century. Early users of AM for wounds and post-surgical applications noted how the membrane seemed to disappear and integrate with the patient’s own tissue without a host reaction. This apparent immune neutrality is a result of mechanisms that suppress and modulate the immune system.
The use of AM was initially limited due to storage challenges associated with use of the fresh tissue. Modern processing methods – including dehydration – have delivered options that have longer shelf lives, can be stored at ambient temperatures, and can be terminally sterilized.
Dehydrated human amniotic membrane (DHAM) is now widely used across clinical specialties as a covering for chronic wounds, burns, incisions and other treatment areas where a protective barrier is needed.
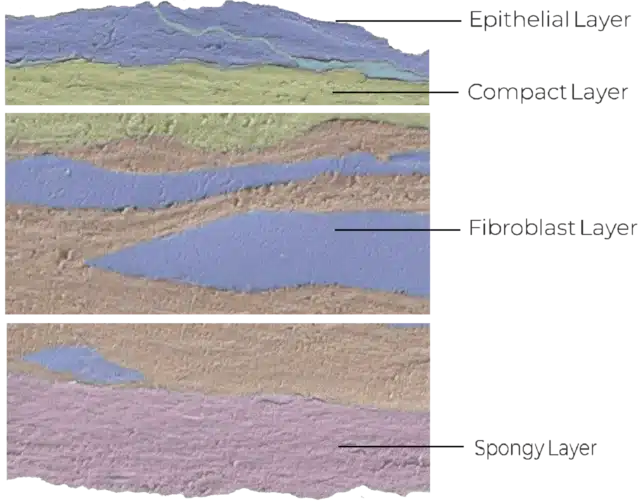
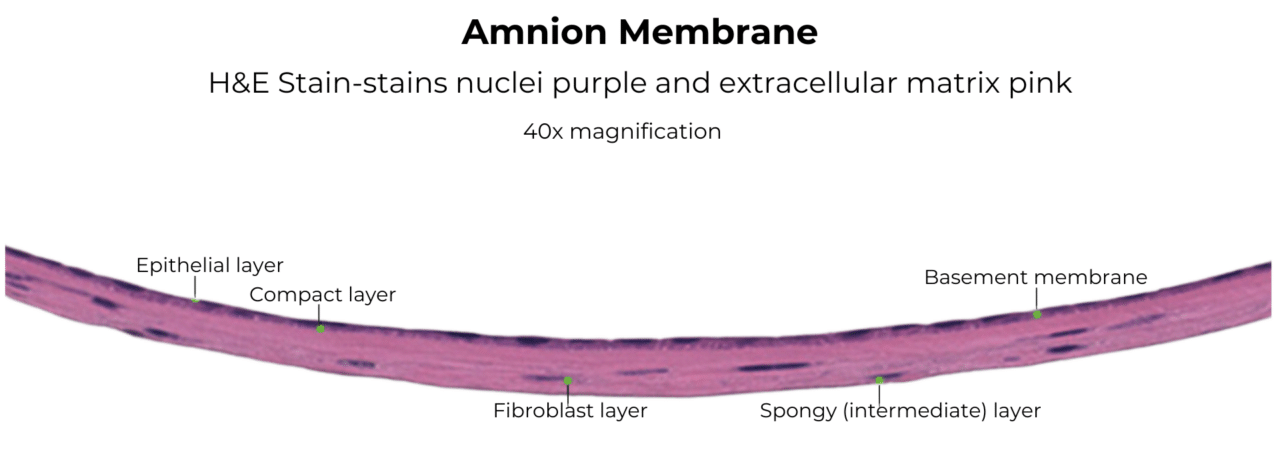
Structure of the Amniotic Membrane
The amniotic membrane forms the innermost layer of the human placenta and acts as a protective barrier for the developing fetus.
A Look Inside VENDAJE®
Components Retained in Amniotic Tissue Allografts
VENDAJE® allografts are prepared using our proprietary BioREtain® process, which was designed to help preserve the natural ECM components, cytokines, and endogenous growth factors within fresh tissue.
Natural Amniotic Components Retained in VENDAJE®:
Scientific analysis of tissue processed using the BioREtain method demonstrates retention of factors key to supporting the wound healing process.
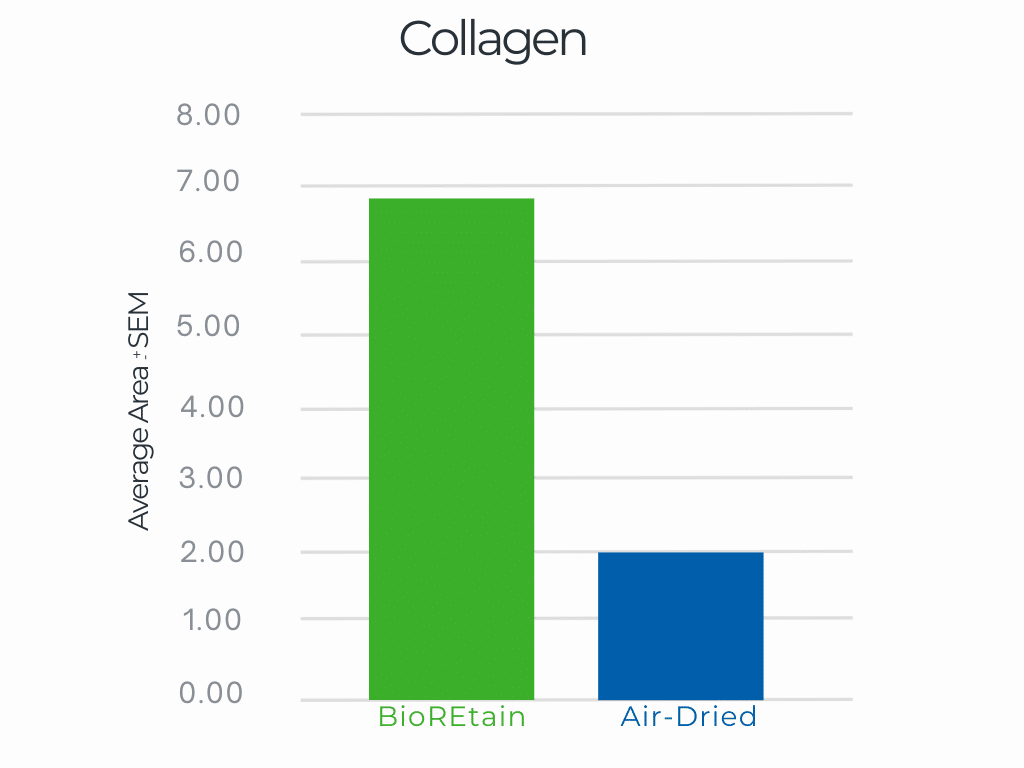
Long strand collagen structure left intact with no cross linking after BioREtain processing. Results are better than even air drying. Long strand collagen promotes a structural environment for wound healing.
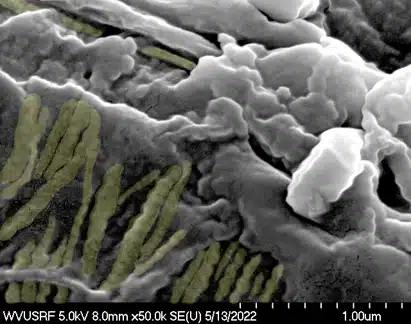

BioREtain processed tissue shows blue staining consistent with the presence of glycosaminoglycans a critical component of the ECM and wound repair.
BioREtain processed tissue shows brown staining consistent with the presence of fibronectin.
Our BioREtain process retains a significant amount of FGF-2
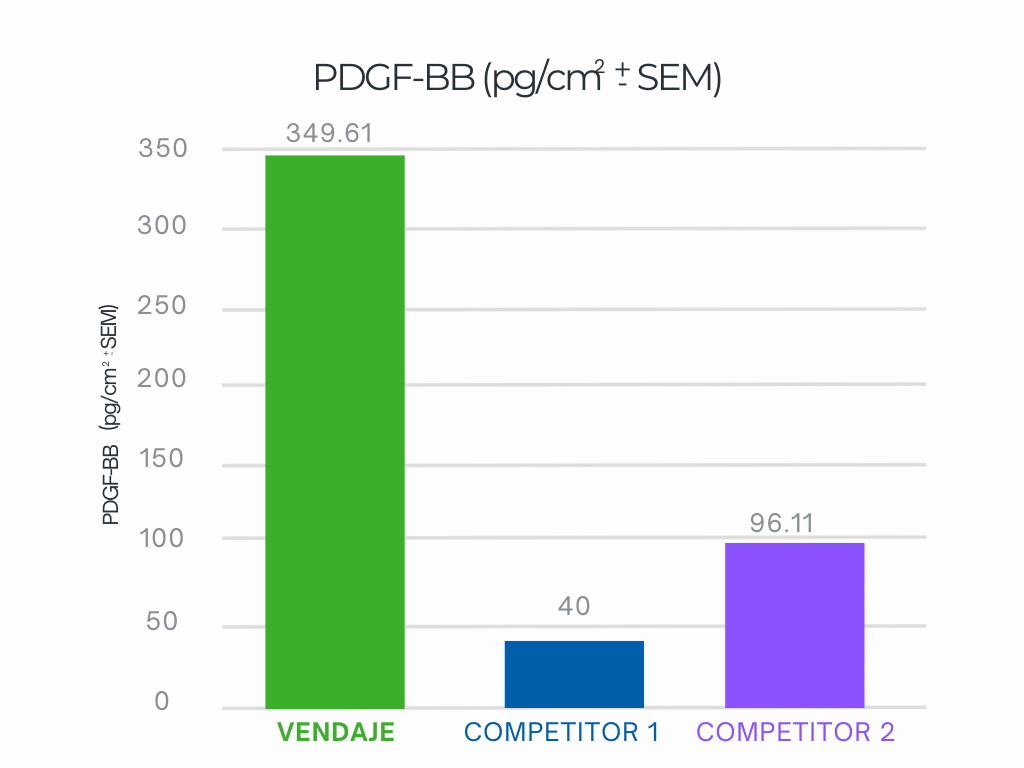
VENDAJE demonstrates nearly 3.6X more PDGF-BB
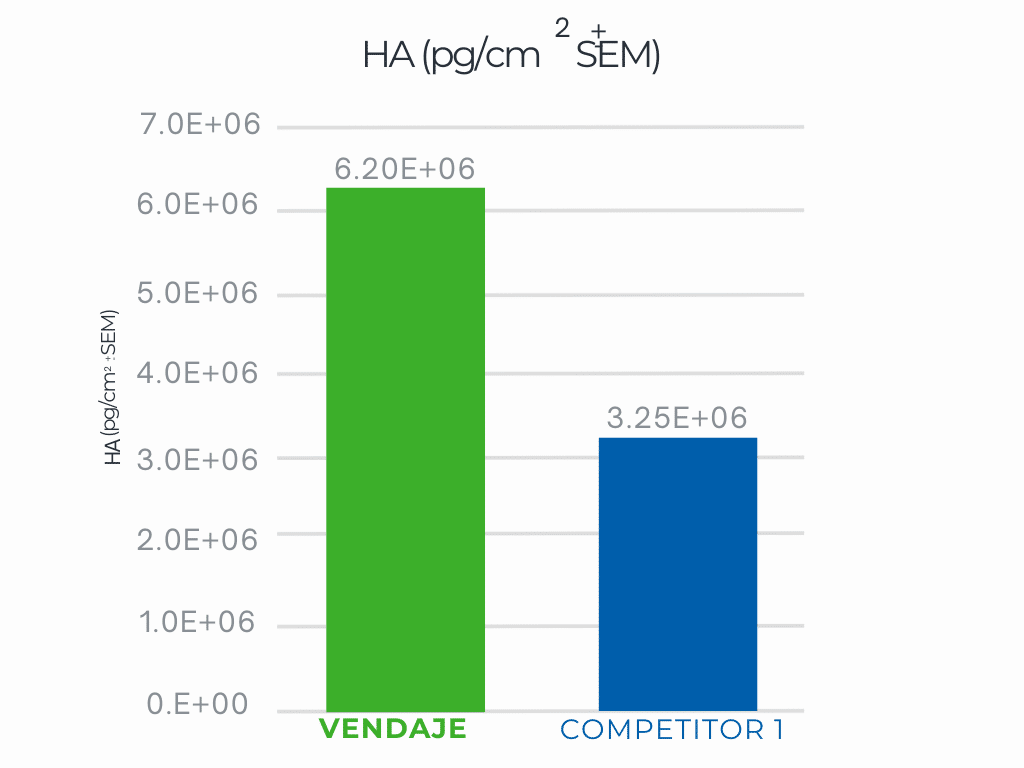
VENDAJE demonstrates significantly more HA
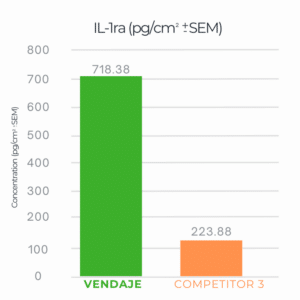
VENDAJE demonstrates more than 3x IL-1ra
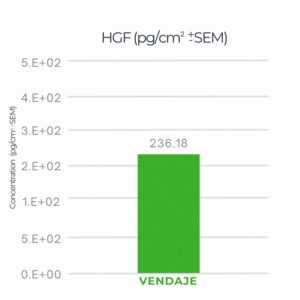
VENDAJE demonstrated a score of 236.18 for HGF
Ordering Information
VENDAJE® allografts are available in a wide range of sizes and shapes for maximum versatility.

| Product SKU | Size |
| 200-004-0202-001 | 2 x 2 cm |
| 200-008-0204-001 | 2 x 4 cm |
| 200-016-0404-001 | 4 x 4 cm |
| 200-024-0406-001 | 4 x 6 cm |
| 200-032-0408-001 | 4 x 8 cm |
| 200-036-0606-001 | 6 x 6 cm |
| 200-080-0810-001 | 8 x 10 cm |
| 200-100-1010-001 | 10 x 10 cm |
| 200-150-1015-001 | 10 x 15 cm |
| 200-200-1020-001 | 10 x 20 cm |
Contact our product experts for more information or to place an order:
Product Hotline:
Local: 954-380-8342
Toll-Free: 1-888-948-BSEM (2736)
Email Inquiries:
orders@biostemtech.com
Care Partner Program
BioStem’s Care Partner Program is one way we show our dedication to supporting providers through every aspect of using our products. Our team is committed to facilitating the best possible customer experience and answering your questions about the coding and reimbursement process.

Have questions or need assistance with reimbursement for VENDAJE
Contact our team of
experienced professionals
Email: reimbursement@biostemtech.com
Reimbursement Hotline (Toll-Free):
1-888-948-BSEM (2736)
Billing Code Support*
*Facilities should select the most appropriate revenue code based on the services provided and internal accounting policies. The information contained herein is not intended as coding advice and is provided for informational purposes only. It represents no statement, promise or guarantee by BioStem Technologies concerning levels of reimbursement, payment, eligibility, charges or that these policies and codes will be appropriate for specific services or products provided or that reimbursement will be made. It is always the providers’ responsibility to determine and submit appropriate codes, charges, modifiers and bills for the services that were rendered. BioStem Technologies recommends that you consult your local CMS MAC or other applicable payor organization with regard to specific reimbursement policies, coverage, documentation and payment.
VENDAJE® is intended for homologous use as a covering and barrier for human tissue. Refer to VENDAJE Instructions for Use for information on safety, usage, and storage.
References
- VENDAJE® Instructions for Use
- Data on File
- Tenehaus M. The Use of Dehydrated Human Amnion/Chorion Membranes in the Treatment of Burns and Complex Wounds. Ann Plast Surg. 2017;78: S11–S13.
- Silini AR, Cargnoni A, Magatti M, Pianta S and Parolini O (2015) The long path of human placenta, and its derivatives, in regenerative medicine. Front. Bioeng Biotechnol.
2015;3:162. - Ramuta TZ, Šket T, Erjavec MS, Kreft ME. Antimicrobial Activity of Human Fetal Membranes: From Biological Function to Clinical Use. Front Bioeng Biotechnol. 2021;9:691522.
- Heckmann N, Auran R, Mirzayan R. Application of Amniotic Tissue in Orthopedic Surgery. Am J Orthoped. 2016;45(7):E421-E425.
- Niknejad H, Peirovi H, Jorjani M et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cells Mater. 2008;15(15):88-99.
- Moore MC, Bonvallet PP, Damaraju SM, et al. Biological characterization of dehydrated amniotic membrane allograft: Mechanisms of action and implications for wound care. J
Biomed Mater Res. 2020;1–8. - Wassmer C-H. Berishvili E. Immunomodulatory Properties of Amniotic Membrane Derivatives and Their Potential in Regenerative Medicine. Curr. Diab. Rep. 2020;20(31).
- Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol. 2014;5(23).
- Moreno SE, Massee M, Koob TJ. Dehydrated Human Amniotic Membrane Inhibits Myofibroblast Contraction through the Regulation of the TGFb‒SMAD Pathway In Vitro. JID
Innov. 2021;1:100020 - Zelen CM, Serena TE, Denoziere G, Fetterolf DE.A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic
foot ulcers. Int Wound J. 2013;10(5):502-507.
VENDAJE®, VENDAJE AC® and VENDAJE OPTIC® are perinatal tissue-derived allografts. Each product is designated as a Human Cell, Tissue, and Cellular and Tissue-Based Product (HCT/P) by the U.S. Food and Drug Administration (FDA), minimally manipulated, and produced in accordance with the FDA regulations for Good Tissue Practices (21 CFR 1270, 1271) in our AATB® accredited lab.
