VENDAJE AC®
Naturally Robust Support
Our BioREtain process is designed to preserve growth factors and cytokines with each VENDAJE allograft—delivering a minimally-manipulated covering.
VENDAJE AC® is intended for use as a wound covering or barrier membrane over acute and/or chronic wounds
Vendaje AC® is a human connective tissue matrix comprised of dehydrated amniotic/chorionic tissue
which is manufactured using our proprietary BioREtain process. This creates a dehydrated human amniotic/chorionic membrane (DHACM) allograft.

VENDAJE AC®
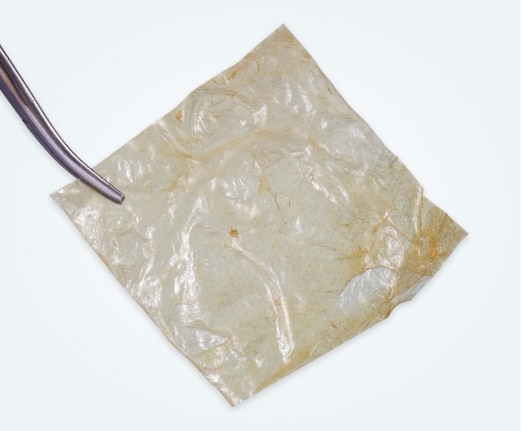
Easy to Use—Easy to Store
VENDAJE AC allografts
- are held in place via hydrostatic tension and may be used in an open incision or placed as a dermal substitute.*
- are aseptically processed and terminally sterilized via e-beam irradiation.
- have a 4-year shelf life and can be stored at ambient temperatures.
*Standard fixation can be used. Reference VENDAJE instructions for use.
The increased thickness, as well as the greater quantity of natural growth factors
resulting from the inclusion of the intermediate and chorion layers, VENDAJE AC is a more robust covering than a single-layer allograft.
Clinicians performing major surgery, as well as those treating complex wounds and severe burns, have explored the use of DHACM as a covering for areas in need of enhanced tissue protection.
Properties of Dehydrated Human Amniotic/Chorionic Membrane

Provides barrier for protection
Terminally sterilized

Helps
prevent
moisture loss
Provides a
structure for
tissue integration

Contains a
range of
growth factors

Naturally contains cytokines
Amniotic tissue is a natural reservoir of growth factors, extracellular matrix (ECM) components, and cytokines.
Multi-layered Support
Placentally-derived human amniotic membrane (AM) is a source of growth factors and cytokines and has successfully been used in regenerative medicine for over a century. Early users of AM for wounds and post-surgical applications noted how the membrane seemed to disappear and integrate with the patient’s own tissue without a host reaction.
The use of AM was initially limited due to storage challenges associated with use of the fresh tissue. Modern processing methods – including dehydration – have delivered options that have longer shelf lives, can be stored at ambient temperatures, and can be terminally sterilized. In recent decades, advances in our understanding of placental tissues and improved processing abilities have resulted in the creation of dehydrated combined amniotic/chorionic membrane (DHACM) products.
DHACM is now widely used where clinicians believe a thicker covering, richer in growth factors and cytokines, is needed. Post-surgical, trauma, burn, and complex wound applications for DHACM have been well-documented in the literature, and new applications continue to be explored.
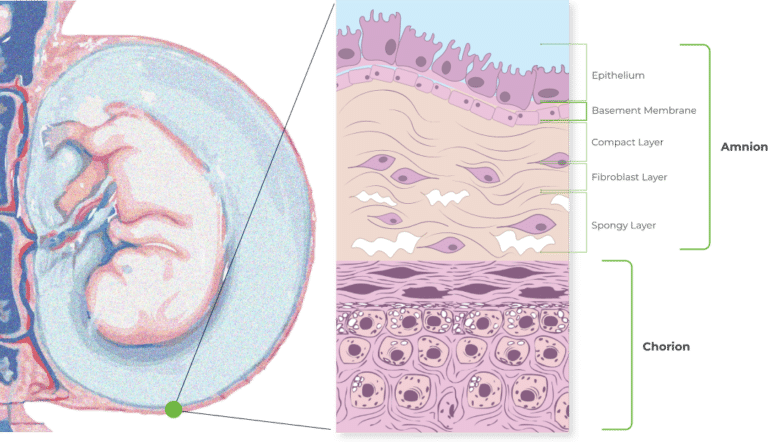
The Combined Amniotic and Chorionic Membranes
The combination of amnion and chorion is thicker than allografts containing amnion alone. It is also richer and more varied in growth factors as well thanks to the higher quantity of growth factors present in the chorion layer*. (*Koob, T. J., Lim, J. J., Zabek, N., & Massee, M. (2015). Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 103(5), 1133-1140).
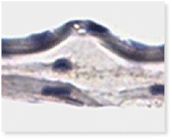
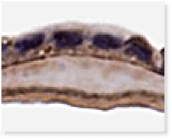
A Look Inside VENDAJE AC®
Why Retain the Intermediate Layer?
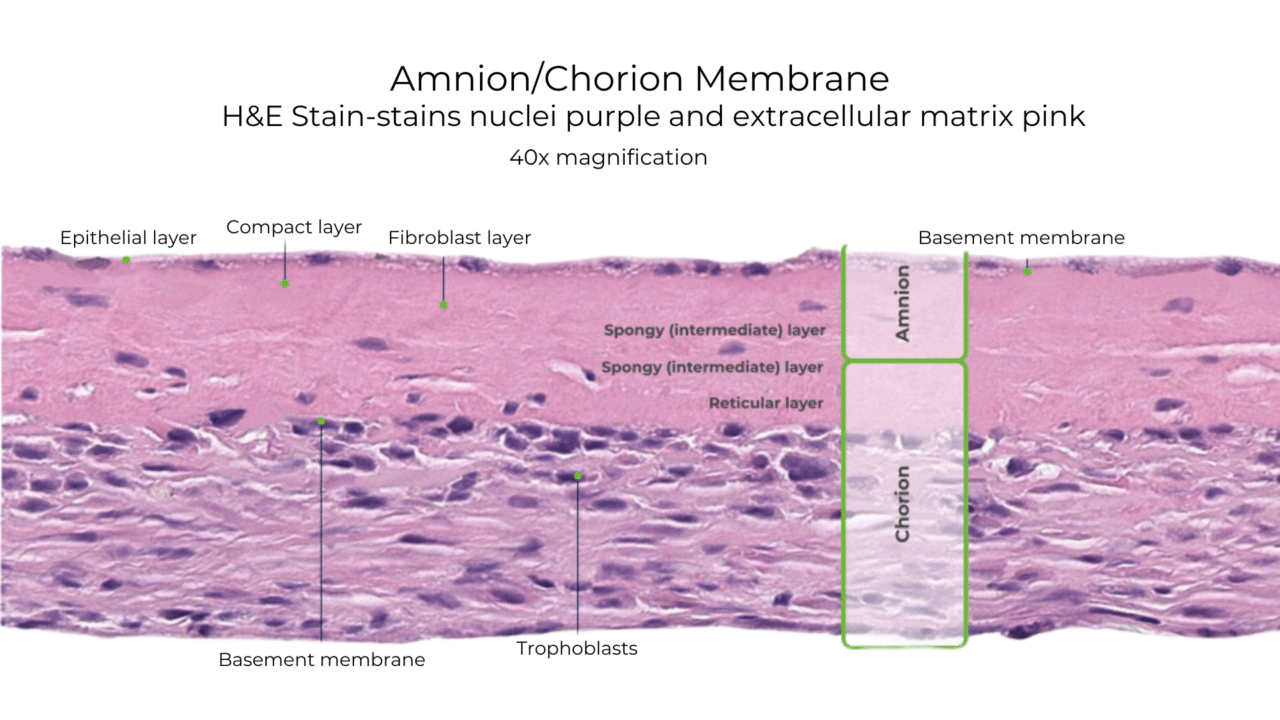
Unlike some other DHACM products, VENDAJE AC also preserves the intermediate layer (IL) between the amnion and chorion. The intermediate layer has its own components, including chemokines, growth factors, interleukins, and protease inhibitors.
Natural Amniotic and Chorionic Components Retained in VENDAJE AC
VENDAJE AC allografts are prepared using our proprietary BioRetain® process, which was designed to help preserve the natural ECM components, cytokines, and endogenous growth factors within fresh tissue.
The following substances were isolated within VENDAJE AC allografts (n=6)*:
Cytokines:
- IL-1 receptor antagonist
Collagens I, III, IV, V, and VI
- Elastin
- Laminin
- Fibronectin
- Proteoglycans
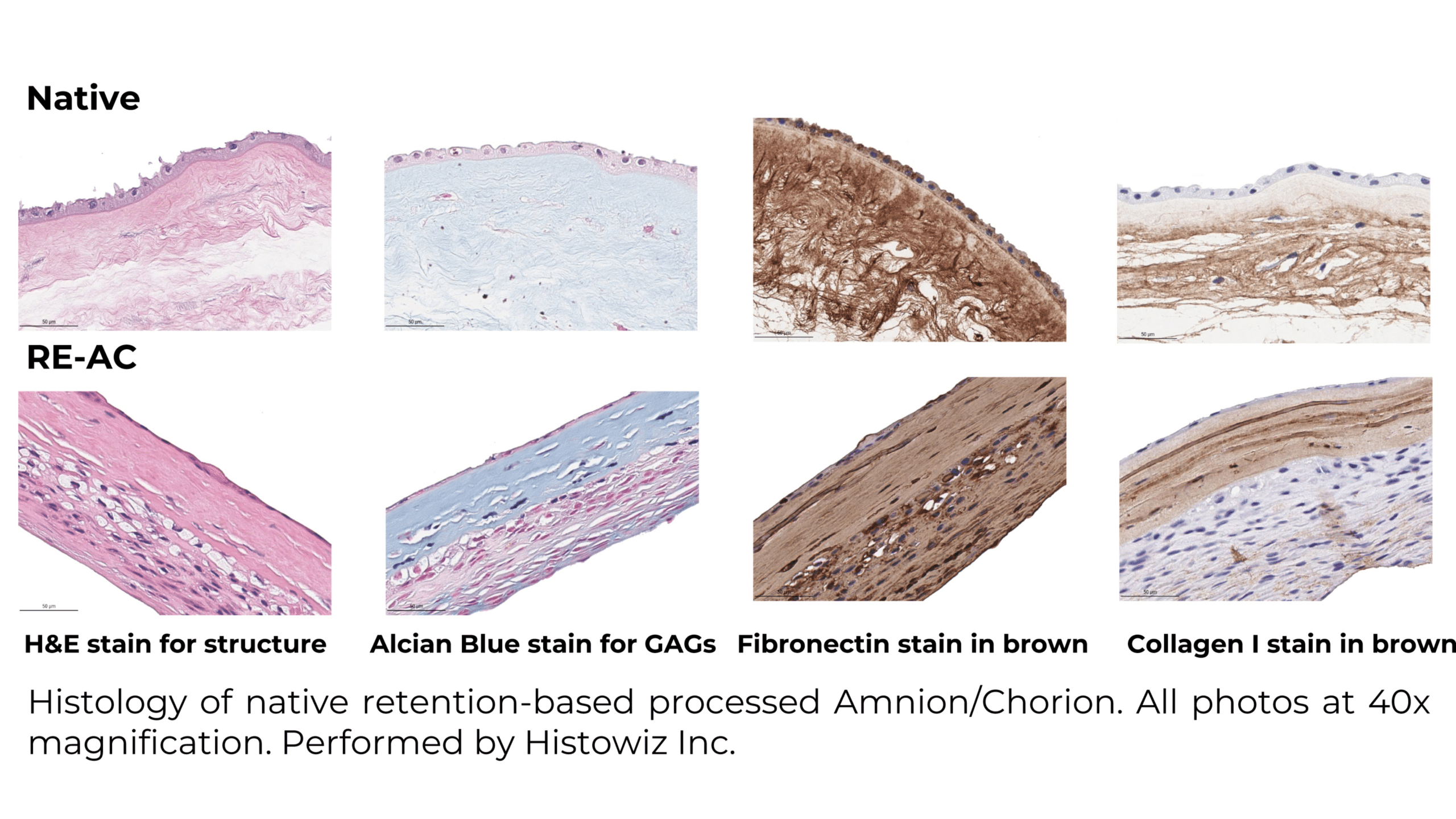
Assessing Treatment Efficiency in Diabetic Foot Ulcers: Retention Processed* Amnion/Chorion Membranes vs. SOC: A Retrospective Analysis
Abstract
Diabetic foot ulcers are a severe complication for diabetic patents, significantly impacting patient quality of life and healthcare systems efficiency. These ulcers often lead to hospitalization and amputation. Traditional Standard of Care (SOC), are inadequate for many patients, necessitating the use of advanced wound care products, such as human placental membranes. This study conducts a retrospective analysis to compare the effectiveness of a human placental amnion/chorion membrane product using retention-based processing (RE-AC) and Standard of Care (SOC) in the treatment modality for chronic diabetic foot ulcers (DFUs).
Methods/Statistical Analysis
The study collected retrospective observational data from electronic health records (EHRS) of patients treated with retention based processed Amnion/Chorion* (RE-AC) as a wound covering at three outpatient wound care centers. Additionally, synthetic control SOC patients were matched from a wound registry using Coarsened Exact Matching (CEM). Patients were categorized into two cohorts based on whether they received RE-AC or SOC.
Key metrics included wound size progression and wound closure. The analysis employed Bayesian Regression and Hurdle Gamma Analysis of Variance (ANCOA) models. These methods allow for the highest statistical power of analyses that are focused on change form a baseline and increase statistical power and precision. They are a more robust approach and offer the opportunity to control potential confounding variables, thereby enhancing the precision of our findings.
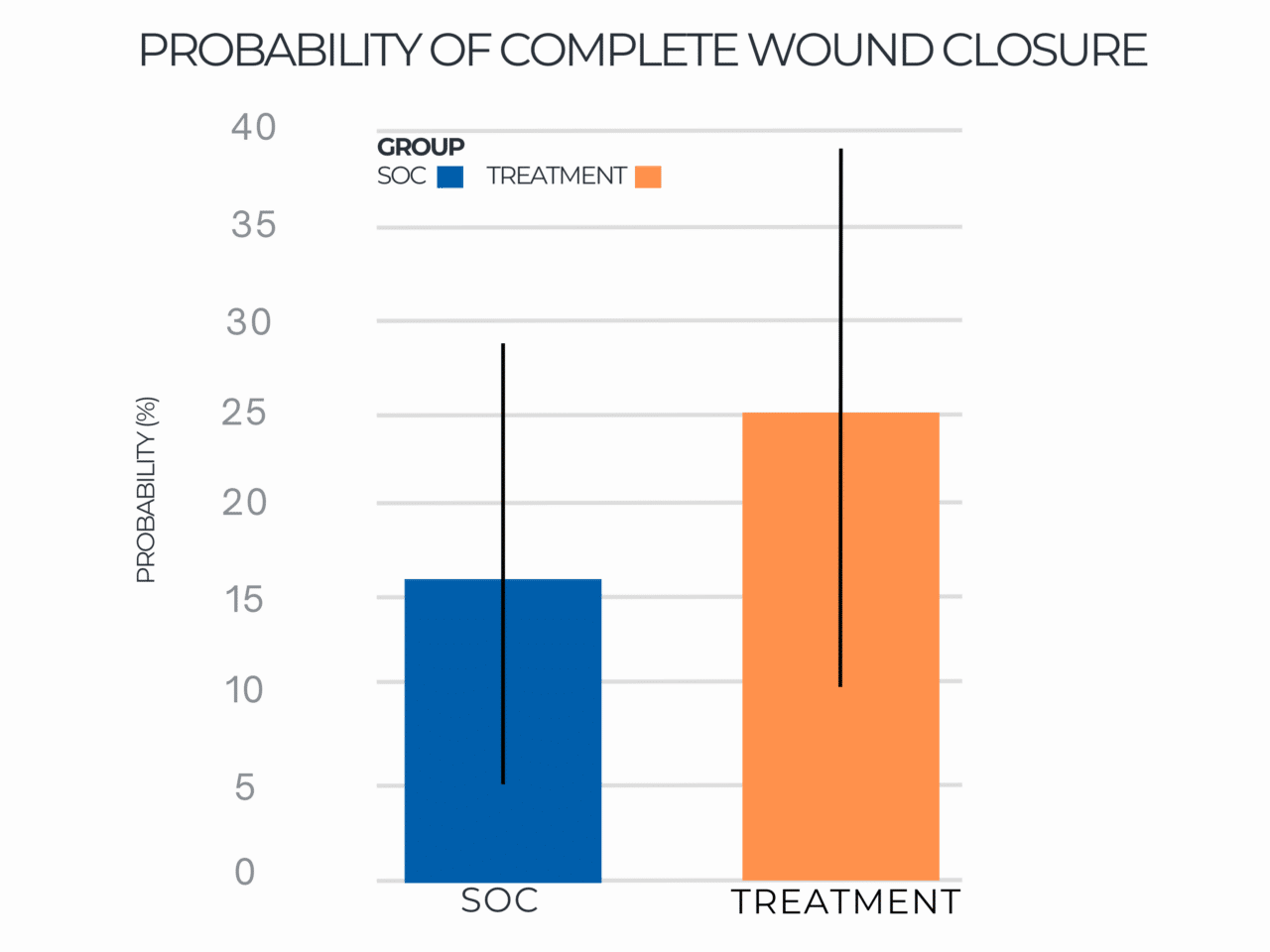
Probability of Complete Wound Closure
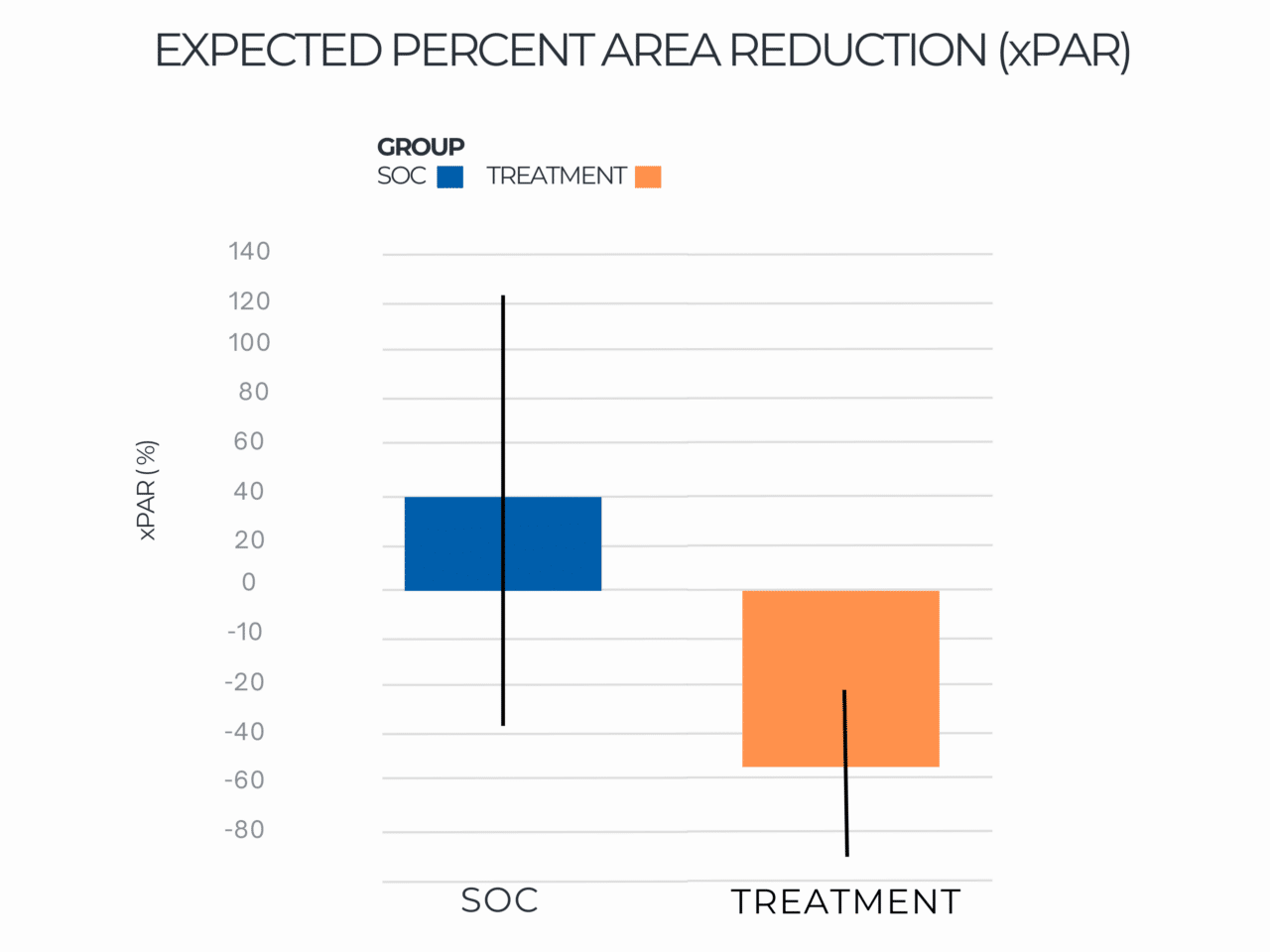
Expected Percent Area Reduction (xPAR)
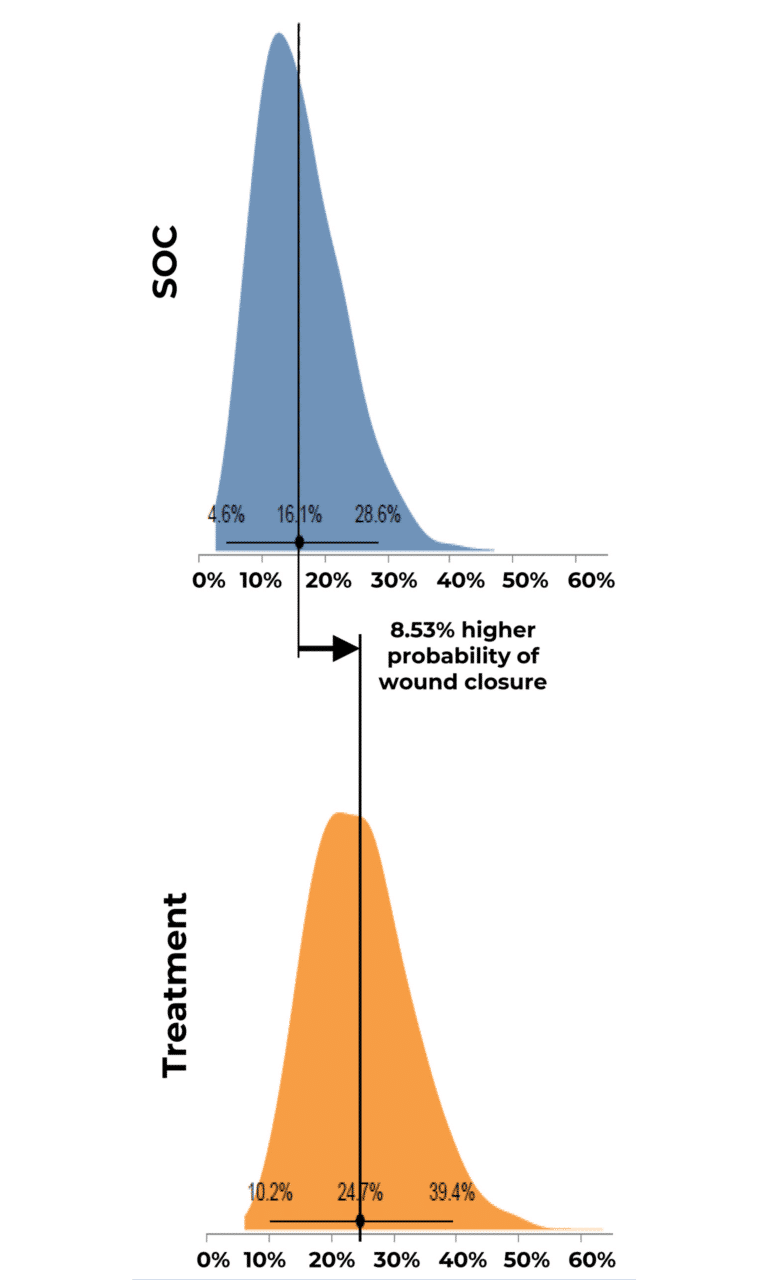
Probability of Complete Wound Closure by Group Distribution
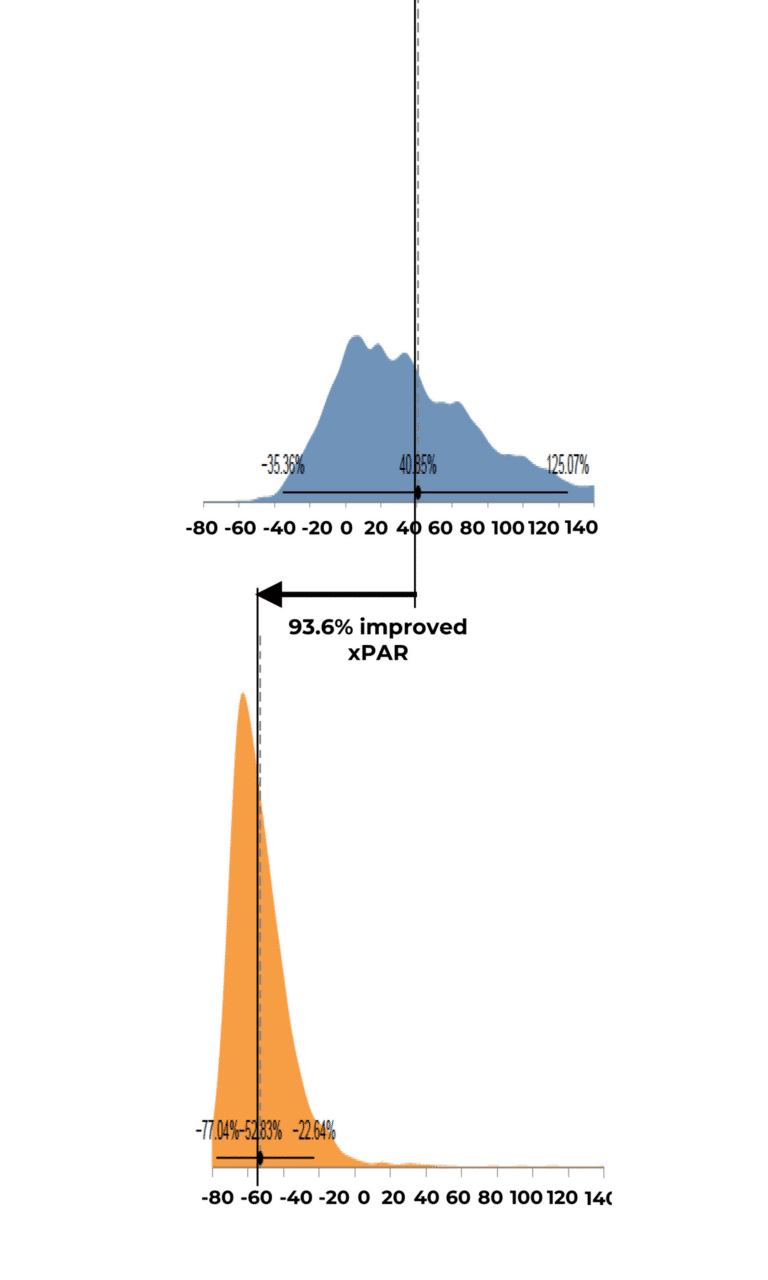
Expected Percent Area Reduction by Group Distribution
Results/Conclusions
Results indicated that use of RE-AC as a covering achieved almost a 10% higher expected wound closure rate compared to SOC at 12 weeks (8.53% (credible interval: 4.50% – 19.7%). Further, for wounds that did not close, RE-AC resulted in a 93.6% (credible interval: 147.7% – 41.6) improvement in expected Percent Area Reduction (xPAR) over the SOC group at 12 weeks. We noted that on average, SOC wounds stalled or grew larger. In terms of a risk ratio comparing the study group with SOC, we found a 52% benefit in the RE-AC group (RR=1.52). The findings suggest that RE-AC is superior to SOC when used as a covering for wound treatment and resulted in an expected Percent Area Reduction by 12 weeks. The benefit likely leads to reduced treatments costs, optimized resource utilization and improved outcomes in the DFU patient population, ultimately resulting in improved patient care.
Histology of VENDAJE AC®
Hyaluronic acid (HA):
Average HA concentration in a VENDAJE AC allograft*: 48,355,062 picograms/cm2
—nearly 8 times higher than our single-layer allograft (*2 Data on File)
Growth factors:
- Epidermal growth factor (EGF)
- Vascular endothelial growth factor (VEGF)
- Basic fibroblast growth factor (bFGF)
- Keratinocyte growth factor (KGF)
The chorionic layer provides a significantly higher amount of growth factors than the amniotic layer, with the exception of EGF.
*This is not a comprehensive list of the substances found within a VENDAJE AC® allograft.
Independent Laboratory Testing Results
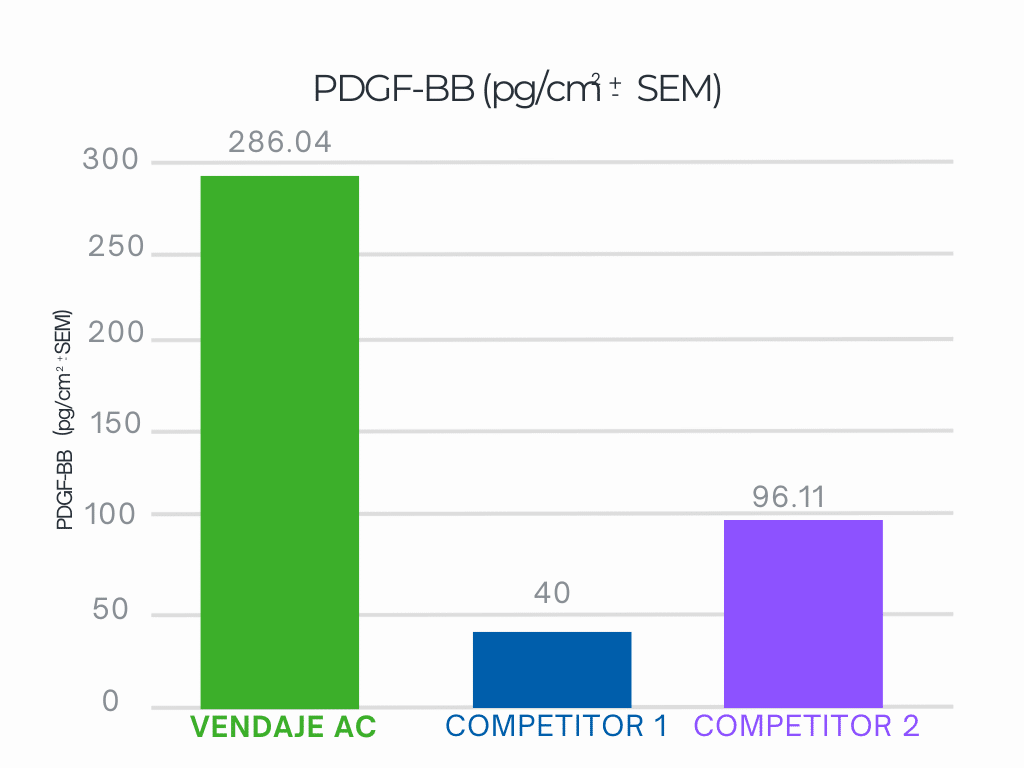
VENDAJE AC demonstrates nearly 3.6X more PDGF-BB
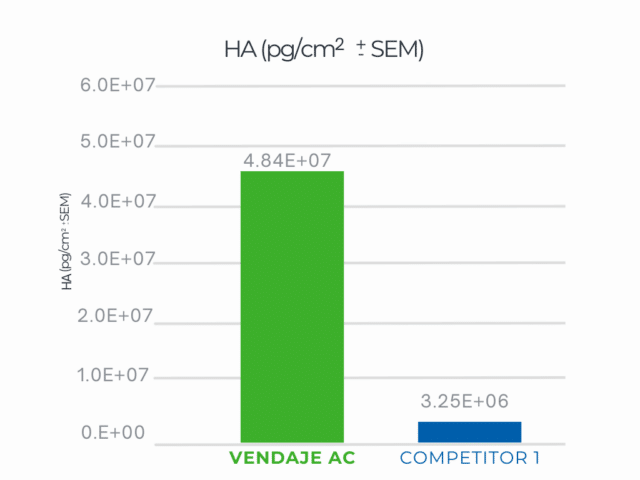
VENDAJE AC demonstrates significantly more HA
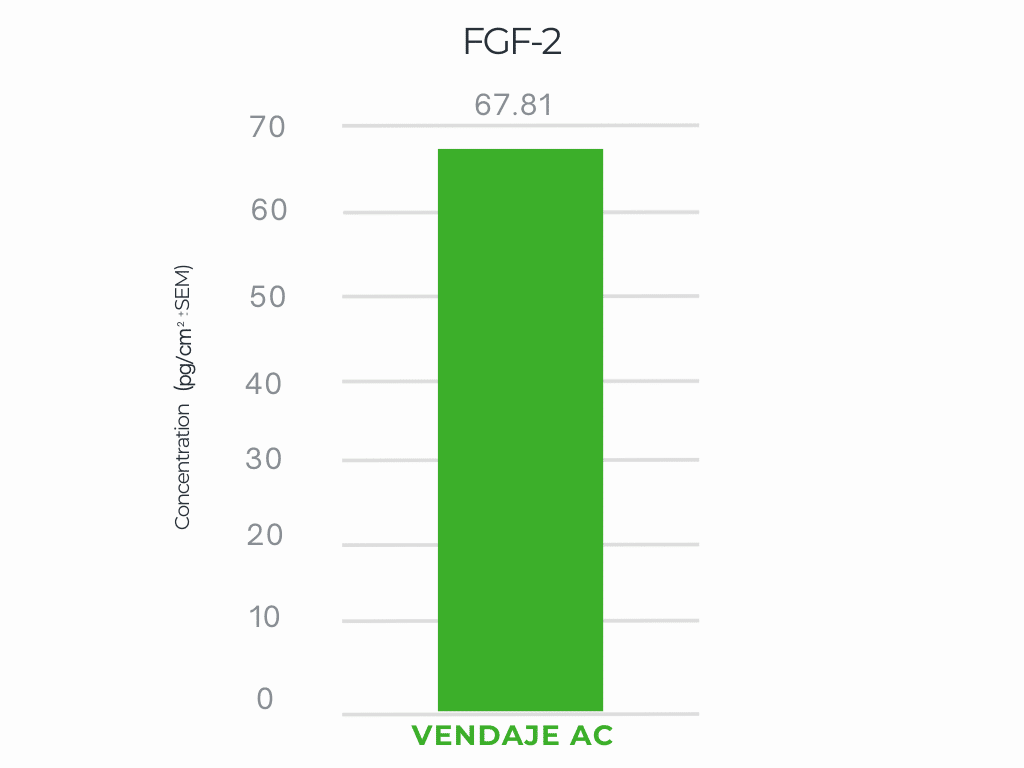
VENDAJE AC demonstrates a significant amount of FGF-2
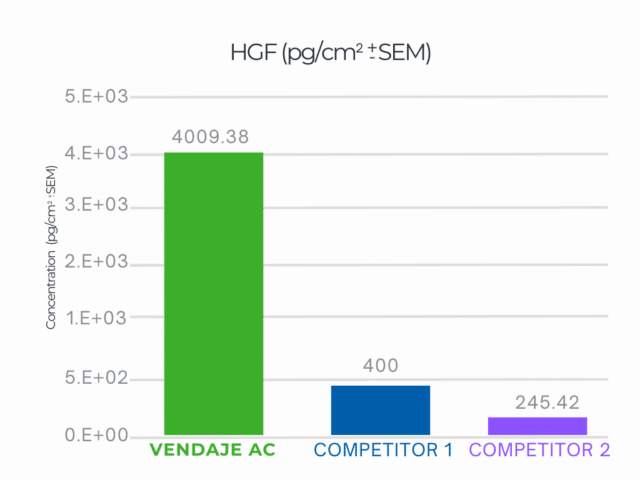
VENDAJE AC demonstrated a 10X higher result than the closest competitor for HGF
Ordering Information
Contact our product experts for more information or to place an order:
Product Hotline:
Local: 954-380-8342
Toll-Free: 1-888-948-BSEM (2736)
Email Inquiries:
orders@biostemtech.com

VENDAJE AC allografts are available in a wide range of sizes and shapes for maximum versatility.
| Product SKU | Size |
| 300-001-0101-001 | 1 x 1 cm |
| 300-004-0202-001 | 2 x 2 cm |
| 300-008-0204-001 | 2 x 4 cm |
| 300-016-0404-001 | 4 x 4 cm |
| 300-024-0406-001 | 4 x 6 cm |
| 300-032-0408-001 | 4 x 8 cm |
| 300-036-0606-001 | 6 x 6 cm |
| 300-080-0810-001 | 8 x 10 cm |
| 300-100-1010-001 | 10 x 10 cm |
| 300-150-1015-001 | 10 x 15 cm |
| 300-200-1020-001 | 10 x 20 cm |
| 300-400-1020-001 | 10 x 20 cm 2 Pack |
| 300-006-0203-001 | 2 x 3 cm |
| 300-009-0303-001 | 3 x 3 cm |
| 300-049-0707-001 | 7 x 7 cm |
Contact our product experts for more information or to place an order:
Product Hotline:
Local: 954-380-8342
Toll-Free: 1-888-948-BSEM (2736)
Email Inquiries:
orders@biostemtech.com
Care Partner Program
BioStem’s Care Partner Program is one way we show our dedication to supporting providers through every aspect of using our products. Our team is committed to facilitating the best possible customer experience and answering your questions about the coding and reimbursement process.

Have questions or need assistance with reimbursement for VENDAJE AC
Contact our team of
experienced professionals
Email: reimbursement@biostemtech.com
Reimbursement Hotline (Toll-Free):
1-888-948-BSEM (2736)
VENDAJE AC® is intended for homologous use as a covering and barrier for human tissue. Refer to VENDAJE Instructions for Use for information on safety, usage, and storage.
References
- VENDAJE AC® Instructions for Use
- Data on File
- Tenehaus M. The Use of Dehydrated Human Amnion/Chorion Membranes in the Treatment of Burns and Complex Wounds. Ann Plast Surg. 2017;78: S11–S13.
- Silini AR, Cargnoni A, Magatti M, Pianta S and Parolini O (2015) The long path of human placenta, and its derivatives, in regenerative medicine. Front. Bioeng Biotechnol. 2015;3:162.
- Lei et al. Priddy LB, Lim JJ, et al. Identification of Extracellular Matrix Components and Biological Factors in Micronized Dehydrated Human Amnion/Chorion Membrane. Adv Wound Care. 2016;6(2):43-53.
- Ramuta TZ, Šket T, Erjavec MS, Kreft ME. Antimicrobial Activity of Human Fetal Membranes: From Biological Function to Clinical Use. Front Bioeng Biotechnol. 2021;9:691522.
- Heckmann N, Auran R, Mirzayan R. Application of Amniotic Tissue in Orthopedic Surgery. Am J Orthoped. 2016;45(7):E421-E425.
- Niknejad H, Peirovi H, Jorjani M et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cells Mater. 2008;15(15):88-99.
- Oesman I, Dhamar Hutami W. Gamma-treated placental amniotic membrane allograft as the adjuvant treatment of unresponsive diabetic ulcer of the foot. Int J Surg Case Rep. 2020;66:313-318.
- Wassmer C-H. Berishvili E. Immunomodulatory Properties of Amniotic Membrane Derivatives and Their Potential in Regenerative Medicine. Curr. Diab. Rep. 2020;20(31).
- Caporusso J, Abdo R, Karr J, Smith M, Anaim A. Clinical experience using a dehydrated amnion/chorion membrane construct for the management of wounds. Wounds. 2019;31(4 Suppl):S19-S27.
- Moore MC, Bonvallet PP, Damaraju SM, et al. Biological characterization of dehydrated amniotic membrane allograft: Mechanisms of action and implications for wound care. J Biomed Mater Res. 2020;1–8.
- Roy, A, Griffiths, S. Intermediate layer contribution in placental membrane allografts. J Tissue Eng Regen Med. 2020;14:1126– 1135.
- Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol. 2014;5(23).
- Moreno SE, Massee M, Koob TJ. Dehydrated Human Amniotic Membrane Inhibits Myofibroblast Contraction through the Regulation of the TGFb‒SMAD Pathway In Vitro. JID Innov. 2021;1:100020
VENDAGE, VENDAJE AC® and VENDAJE OPTIC® are perinatal tissue-derived allografts. Each product is designated as a Human Cell, Tissue, and Cellular and Tissue-Based Product (HCT/P) by the U.S. Food and Drug Administration (FDA), minimally manipulated, and produced in accordance with the FDA regulations for Good Tissue Practices (21 CFR 1270, 1271) in our AATB® accredited lab.