VENDAJE OPTIC®
Feather-Light and Powerful
Our BioREtain process is designed to preserve more growth factors and cytokines with each VENDAJE allograft—delivering a minimally-manipulated covering.
VENDAJE OPTIC® is Intended for Homologous use as a Protective Covering.
Vendaje OPTIC® is an ultra-thin, ultra-light human connective tissue matrix comprised of amniotic tissue
which is processed using our proprietary BioRetain process.
This process creates a dehydrated human amniotic membrane

VENDAJE OPTIC® is intended for homologous use as a protective covering.
Easy to Use—Easy to Store
VENDAJE OPTIC allografts
- can be applied directly to the eye without the use of additional hardware or sutures.
- have no orientation issues—can be placed with either side facing down.
- are aseptically processed and terminally sterilized via e-beam irradiation.
- have a 4-year shelf life and can be stored at ambient temperatures.
The Intrinsic Properties of Dehydrated Human Amniotic Membrane (DHAM)
make VENDAJE OPTIC a versatile option for a wide variety of topical covering applications.
Properties of Dehydrated Human Amniotic/Chorionic Membrane

Provides barrier for protection
Terminally sterilized

Helps
prevent
moisture loss
Provides a
scaffold for
tissue integration

Contains a
range of
growth factors

Naturally contains cytokines
Amniotic tissue is a natural reservoir of growth factors, extracellular matrix (ECM) components, and cytokines which are known to support the body’s natural healing processes.
A Natural Foundation for Wound Care
Placentally-derived human amniotic membrane (AM) is a potent source of pro-healing growth factors and cytokines and has successfully been used in regenerative medicine for over a century. Early users of AM for wounds and post-surgical applications noted how the membrane seemed to disappear and integrate with the patient’s own tissue without a host reaction. This apparent immune neutrality is a result of mechanisms that suppress and modulate the immune system.
The use of AM was initially limited due to storage challenges associated with use of the fresh tissue. Modern processing methods – including dehydration – have delivered options that have longer shelf lives, can be stored at ambient temperatures, and can be terminally sterilized. These advances also allow manufacturers to create thinner coverings that are suitable for delicate ophthalmic use.
Today, dehydrated human amniotic membrane (DHAM) continues to be widely used and studied as an ophthalmic covering across a range of topical applications.
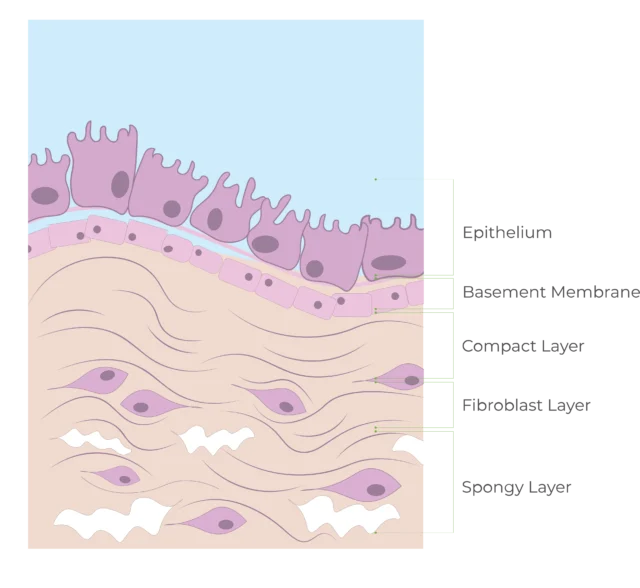
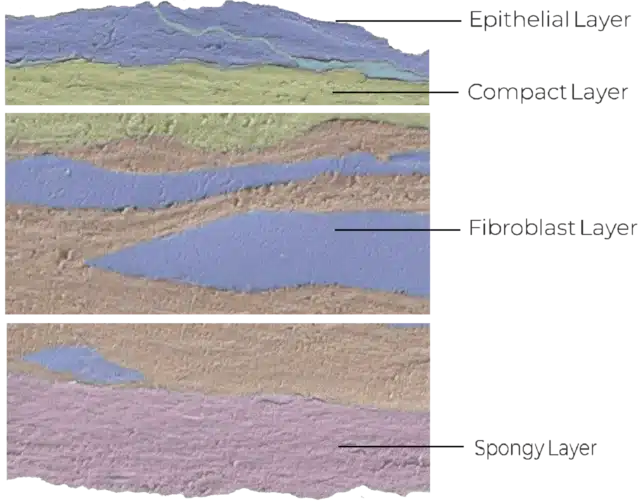
Structure of the Amniotic Membrane
The amniotic membrane forms the innermost layer of the human placenta and acts as a protective barrier for the developing fetus.
Interestingly, the basement membranes of fresh AM and the conjunctiva of the eye share an identical distribution of components (laminin-1, laminin-5, fibronectin, and type VII collagen).
Natural Amniotic Components Retained in VENDAJE OPTIC®
VENDAJE OPTIC allografts are prepared using our proprietary BioRetain® process, designed to help preserve the natural ECM components, cytokines, and endogenous growth factors within fresh tissue.
The following substances were isolated within VENDAJE allografts (n=6)*:
Cytokines:
- Interleukin IL-1ra
This substance naturally inhibits pro-inflammatory effects of IL-1.
Extracellular matrix (ECM) components:
- Collagens I, III, IV, V, and VI
- Elastin
- Laminin
- Fibronectin
The ECM of amniotic membrane provides mechanical protection and functional support for cell attachment, proliferation, and migration.
Hyaluronic acid (HA):
HA suppresses the expression of TGF-β1, β2, and β3, as well as TGF-receptor expression, providing an anti-fibrogenic effect.
Growth factors:
- Epidermal growth factor (EGF)
- Vascular endothelial growth factor (VEGF)
- Basic fibroblast growth factor (bFGF)
- Keratinocyte growth factor (KGF)
*This is not a comprehensive list of the substances found within VENDAJE OPTIC.
Ordering Information


VENDAJE OPTIC allografts are available in a range of sizes for maximum versatility.
| Product SKU | Size |
| 500-050-0008-001 | 8 mm |
| 500-079-0010-001 | 10 mm |
| 500-113-0012-001 | 12 mm |
Contact our product experts for more information or to place an order:
Product Hotline:
Local: 954-380-8342
Toll-Free: 1-888-948-BSEM (2736)
Email Inquiries:
orders@biostemtech.com
Care Partner Program
BioStem’s Care Partner Program is one way we show our dedication to supporting providers through every aspect of using our products. Our team is committed to facilitating the best possible customer experience and answering your questions about the coding and reimbursement process.

Have questions or need assistance with reimbursement for VENDAJE OPTIC?
Contact our team of
experienced professionals:
Reimbursement Hotline (Toll-Free):
1-888-948-BSEM (2736)
VENDAJE OPTIC® is intended for homologous use as a covering and barrier for human tissue. Refer to VENDAJE Instructions for Use for information on safety, usage, and storage.
References
- VENDAJE OPTIC Instructions for Use
- Data on File
- Mimouni M, Trinh T, Sorkin N, Cohen E, Santaella G, Rootman DS, Slomovic AR, Chan CC. Sutureless dehydrated amniotic membrane for persistent epithelial defects. Eur J Ophthalmol. 2021;22:11206721211011354. Mead OG, Tighe S, Tseng SCG. Amniotic membrane transplantation for managing dry eye and neurotrophic keratitis. Taiwan J Ophthalmol. 2020;10(1):13-21. Published 2020.
- Tseng SC. Amniotic membrane transplantation for ocular surface reconstruction. Biosci Rep. 2001;21(4):481-9.
- American Academy of Ophthalmology. “In-Office Use of Amniotic Membrane.” https://www.aao.org/eyenet/article/in-office-use-of-amniotic-membrane. Published Feb 2015.
- Tenehaus M. The Use of Dehydrated Human Amnion/Chorion Membranes in the Treatment of Burns and Complex Wounds. Ann Plast Surg. 2017;78: S11–S13.
- Silini AR, Cargnoni A, Magatti M, Pianta S and Parolini O (2015) The long path of human placenta, and its derivatives, in regenerative medicine. Front Bioeng Biotechnol. 2015;3:162.
- Ramuta TZ, Šket T, Erjavec MS, Kreft ME. Antimicrobial Activity of Human Fetal Membranes: From Biological Function to Clinical Use. Front Bioeng Biotechnol. 2021;9:691522.
- Heckmann N, Auran R, Mirzayan R. Application of Amniotic Tissue in Orthopedic Surgery. Am J Orthoped. 2016;45(7):E421-E425.
- Niknejad H, Peirovi H, Jorjani M et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cells Mater. 2008;15(15):88-99.
- Moore MC, Bonvallet PP, Damaraju SM, et al. Biological characterization of dehydrated amniotic membrane allograft: Mechanisms of action and implications for wound care. J Biomed Mater Res. 2020;1–8.
- Wassmer C-H. Berishvili E. Immunomodulatory Properties of Amniotic Membrane Derivatives and Their Potential in Regenerative Medicine. Curr. Diab. Rep. 2020;20(31).
- Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol. 2014;5(23).
- Moreno SE, Massee M, Koob TJ. Dehydrated Human Amniotic Membrane Inhibits Myofibroblast Contraction through the Regulation of the TGFb‒SMAD Pathway In Vitro. JID Innov. 2021;1:100020
- Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea. 1999;18(1):73-9.
VENDAJE®, VENDAJE OPTIC®, and VENDAJE AC® are perinatal tissue-derived allografts. Each product is designated as a Human Cell, Tissue, and Cellular and Tissue-Based Product (HCT/P) by the U.S. Food and Drug Administration (FDA), minimally manipulated, and produced in accordance with the FDA regulations for Good Tissue Practices (21 CFR 1270, 1271) in our AATB® accredited lab.
